Our Research
|
Temporal association memory
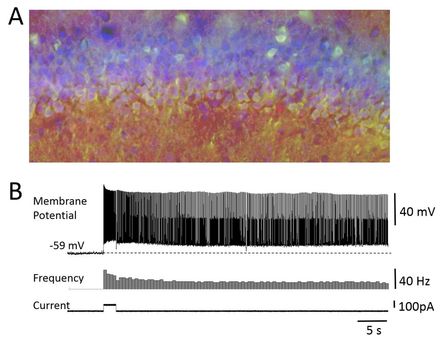
The ability to associate events that are temporally apart, requires an intact hippocampus. Our current major focus is on the roles of transient receptor potential cation (TRPC) channels in temporal association memory. TRPC channels are abundant in hippocampal principal cells (Fig. 1A). We have recently shown that TRPC channels modulate dynamical properties of hippocampal pyramidal cells known as persistent firing (Fig. 1B). Computational simulations have indicated that TRPC channels play crucial roles in shaping neural network activity, allowing persistent firing to co-exist with oscillatory dynamics. We plan to further our understandings of the contributions of TRPC channels on behavioral levels using TRPC channel manipulations in vivo. |
|
Spatial navigation
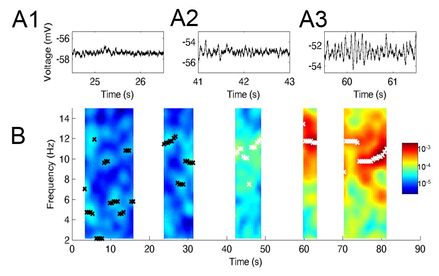
Grid cells in the medial entorhinal cortex (MEC) are believed to support spatial navigation. It has been proposed that grid cell firing emerges through path integration, which is the ability to update spatial representation by using idiothetic cues. MEC layer II has mainly two different types of cells: pyramidal and stellate cells. However, the cellular mechanisms supporting path integration and grid cell formation remain unknown. Oscillatory properties of MEC layer II neurons and persistent firing may support path integration (Fig. 2). We have shown that properties of MEC layer II neurons such as the spike adaptation ratio are different along the dorso-ventral axis, indicating these cellular properties may underlie differences in grid cell spacing. We further continue this line of research to identify cellular mechanism of path integration from cellular to behavioral levels. |
|
Memory encoding and consolidation
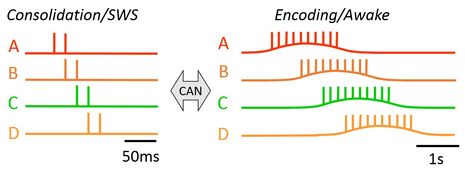
It has been suggested that the MTL supports memory formation through two distinct processes: encoding and consolidation. Hippocampal neurons switch their firing mode from "place cell" or "time cell" activity during encoding to the temporally compressed "replay" activity during consolidation. The prevailing theory suggests that the level of the neuromodulator acetylcholine triggers this transition of operational modes. Our theoretical and computational work suggests that cholinergic modulations of intrinsic cellular properties, including the TRPC and potassium channels, may support the transition. |
Dr. Motoharu Yoshida | AG Cognitive Neurophysiology | Leibniz Institute for Neurobiology | German Center for Neurodegenerative Diseases | Magdeburg